SYNMET – Solar Combined ZnO-Reduction and CH4-Reforming
Funding source: external page BfE - Swiss Federal Office of Energy, Baugarten Foundation, external page Paul Scherrer Institute
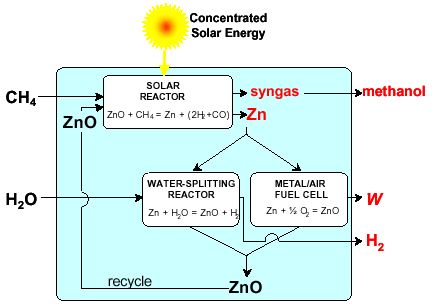
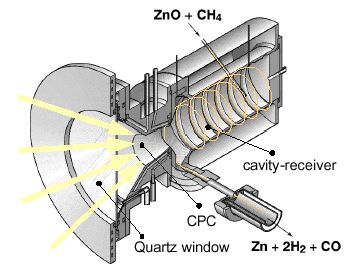
Background – The conversion of solar energy into storable and transportable chemical fuels is investigated by means of thermochemical processes using high-temperature solar process heat. The SynMet-process, i.e. the solar combined reforming of CH4 and reduction of ZnO is considered for co-producing Zn and synthesis gas (syngas). The advantage would be twofold: (1) the reforming of NG in the absence of catalysts may be made to produce high quality syngas with a H2 to CO molar ratio of 2, which is especially suitable for methanol synthesis; (2) CO2 emissions derived in the traditional carbothermic reduction and reforming processes are avoided. The technical feasibility of the SynMet-process was demonstrated using the 5 kW solar reactor.
Aims
- Modelling of solar reactor using CFD and Monte Carlo ray-tracing simulations.
- Establish optimal operating conditions for maximum exergy efficiency.

Project-related Publications