Solar Ammonia - Solar-driven thermochemical production of ammonia via a metal nitride cycle
Partners: external page Prof. María-Elena Gálvez (Sorbonne Université), external page Prof. Greta Patzke (University of Zurich)
Doctoral Student: Daniel Notter
Project Head: Prof. Aldo Steinfeld
Background – Ammonia is among the most important chemical commodities worldwide as it serves as the precursor of agricultural fertilizes. In addition, ammonia can potentially serve as a carbon-free fuel for the transportation sector. Ammonia is conventionally produced by the Haber-Bosch process, which involves the catalytic reaction of N2 and H2 at 400-500 °C and 200-300 bars. Despite these harsh operating conditions, single pass yields do not exceed 20-25 %. Currently, the ammonia production industry is responsible for over 1 % of the global anthropogenic greenhouse gas emissions, mainly derived from the use of fossil fuels required to produce H2 and to supply the energy for the compression of the gas feed.
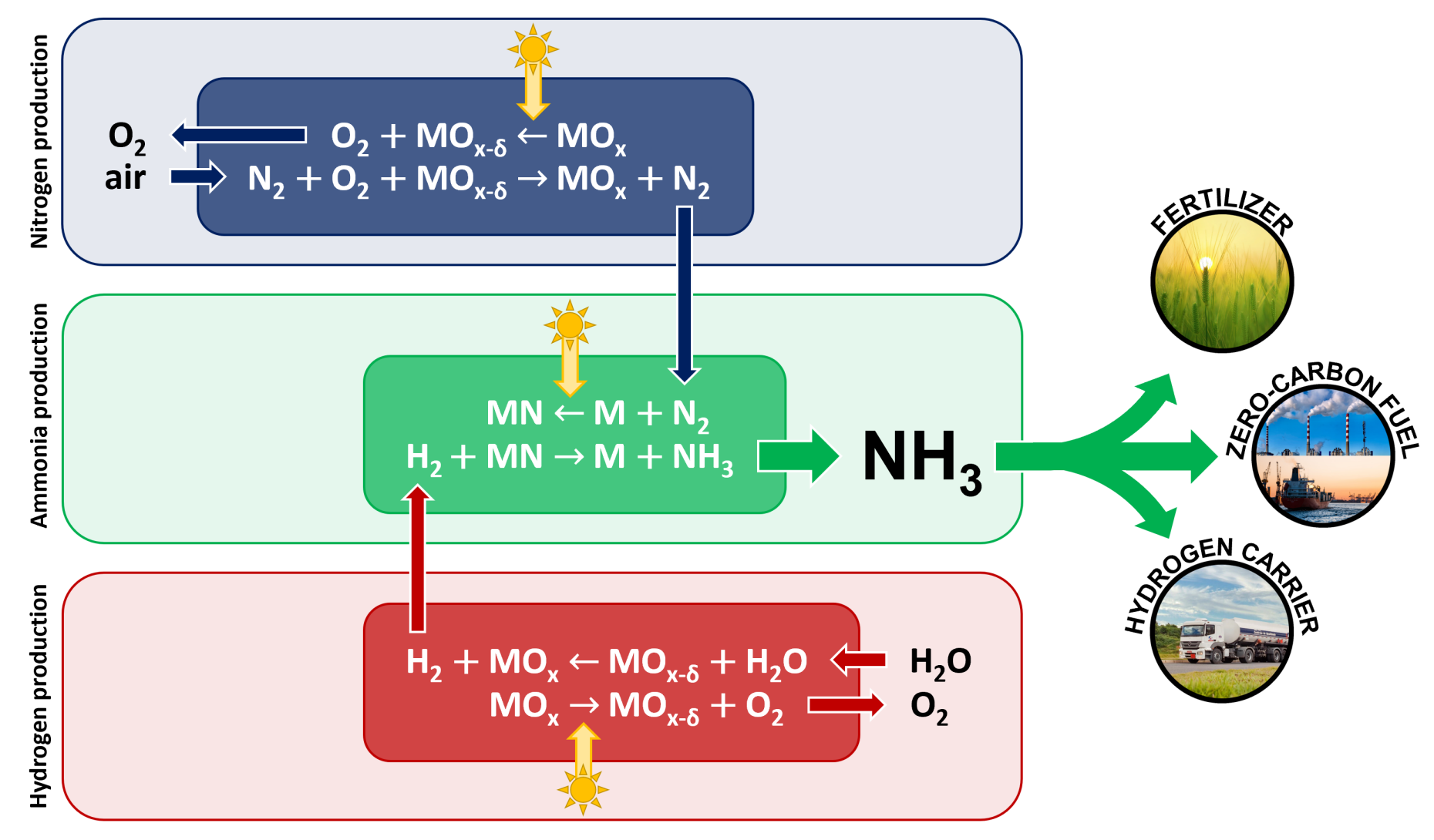
Objective - The objective of this project is to develop a thermochemical cyclic process to produce ammonia, driven by concentrated solar process heat, that mitigates the greenhouse gas emissions associated with the Haber-Bosch process. The two-step cycle is schematically shown in the figure above. The first, endothermic step consists of the nitridation of a metal, which proceeds to form a metal nitride. The second, exothermic step consists of the hydrogenation of the metal nitride to produce ammonia and the metal; the latter is recycled to the first step. By separating the high-temperature N2 cleavage from the ammonia synthesis step, much lower pressures are necessary. This simplifies the design and operation of ammonia production plants, opening the pathway to smaller scale, decentralized plants.
Research tasks:
• Thermodynamic analysis and computational screening for metal nitrides.
• Experimental thermogravimetric analysis and material characterization.
• Experimental investigation of a lab-scale packed bed reactor.