Ammonia Production via a 2-Step Al2O3/AlN Thermochemical Cycle
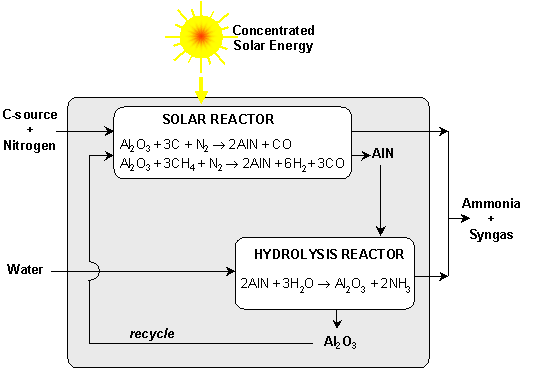
The production of ammonia via a 2-step cyclic process is proposed as an alternative to its conventional production by the Haber-Bosch process. The first, endothermic step is the production of AlN by carbothermic reduction of Al2O3 in a N2 atmosphere at above 1500°C. The second, exothermic step is the steam-hydrolysis of AlN to produce NH3 and reform Al2O3; the latter is recycled to the first step. Further, the endothermic reduction step can be carried out using concentrated solar energy as the source of process heat. Figure 1 illustrates the proposed cycle.
1st step: Al2O3 + 3C + N2 → 2AlN + 3CO (1a)
Al2O3 + 3CH4 + N2 → 2AlN + 6H2+ 3CO (1b)
2nd step: 2AlN + 3H2O → Al2O3 + 2NH3 (2)
The proposed 2-step process offers the following four-fold advantages:
- it eliminates the need for high pressure, minimizing costs and safety concerns;
- it eliminates the need for catalysts; minimizing costs associated with their production and recycling;
- it eliminates the need for hydrogen as feedstock, reducing energy consumption and associated CO2 emissions.
- It eliminates concomitant CO2 emissions derived from fossil-fueled endothermic processes.
Project-related Publications