EU-Project SOLHYCARB – Solar Cracking of Methane
Funding source: external page European Union
Partners: external page CNRS (France), N-GHY (France), CREED (France), external page DLR (Germany), external page Weizmann (Israel), external page Abengoa Solar (Spain), external page Timcal (Belgium), external page CERTH (Greece), external page PSI (Switzerland)
Background – The SOLHYCARB proposal addresses the exploration of an unconventional route for potentially cost effective hydrogen production with solar energy. The novel process thermally decomposes natural gas (NG) in a high temperature chemical reactor heated by concentrated solar energy. This process results in two products: a H2-rich gas and a high-value nano-material, carbon black (CB). H2 is thus produced with renewable energy. Solar energy is stored as a transportable fuel. The fuel has zero CO2 emission: carbon as opposed to CO2 is sequestered. Fossil fuels are saved, and CB is synthesized.
This solar process is described in Figure 1.
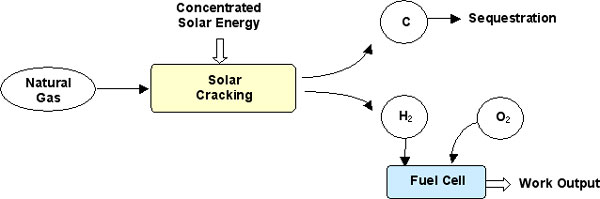
This process permits the following:
- Production of a powerful energy carrier (Hydrogen) with solar energy
- Storage of solar energy
- Avoidance of CO2 emission because Carbon is sequestrated
- Synthesis of marketable Carbon Black (CB)
- Saving of fossil fuel with respect to the production of H2 and CB by conventional methods
Impact – Potential impacts on CO2 emission reduction and energy saving are respectively: 14 kg CO2 avoided and 277 MJ per kg H2 produced, with respect to conventional NG steam reforming and CB processing. Projected cost of H2 for large-scale solar plants depends on the price of CB: about 14 €/GJ for the lowest CB grade sold at 0.66 €/kg and decreasing to 10 €/GJ (i.e. the current reference price of H2 from steam reforming with CO2 sequestration) for CB at 0.8 €/kg. In summary, this solar process based on NG decarbonisation is a pathway towards a more sustainable production of hydrogen and Carbon Black.
Scientific and technological objectives – The project aims at co-producing hydrogen-rich gas and high-grade carbon black from natural gas cracking using concentrated solar energy in a high temperature solar reactor. It includes the design, construction and testing of innovative solar reactor prototypes of 1-10 kW and a pilot reactor of 50 kW for operating conditions at 1500-2300 K and 1 bar. In the first step of the project, two different prototypes based on different concepts of solar receiver/reactor (direct and indirect heating concepts) will be developed and studied. A critical analysis of the results (from experiments and modelling) will lead to the choice of the best reactor concept (direct or indirect heating) suitable for solar methane splitting. Based on the concept retained, a 50 kW power pilot reactor will be developed. The targeted results are: methane decomposition yields over 80%, hydrogen content in the off-gas over 75%, and CB properties equivalent to industrial products. This experimental work is highly combined with advanced reactor modelling, study of separation unit operations and determination of CB properties for applications in batteries and polymers, thus resulting in the necessary information for someday scaling up the process. Decentralized and centralized commercial solar chemical plants will be designed for 50 kWthand 10/30 MWth.
The objectives of the SOLHYCARB project are:
- the design, modelling, and construction of innovative solar reactors;
- the testing and performance evaluation of the solar reactors;
- the analysis of the produced gas and its purification to obtain hydrogen;
- the measurement of CB characteristics and properties;
- the technical-economical design of decentralized and large scale solar processes (based on the solar tower concept).
Measurable objectives of the project:
Solar reactors: Lab-scale 1-10 kW and pilot-scale 50 kW
Reactor operating temperature: 1500-2300 K
Methane decomposition: 80%
Hydrogen in the off-gas: 75%
H2 and CB Production: 3 standard m3/h H2 and 1 kg/h C
Produced CB properties: Equivalent to industrial CB grades
Solar chemical plant design: Cost of H2 versus CB market price
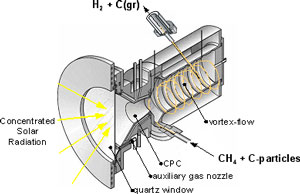
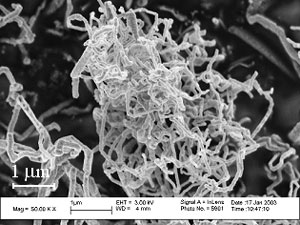
Previous work on solar reactor technology – The solar chemical reactor for the thermal cracking of CH4 is shown schematically in Fig. 2. It features a vortex flow of CH4 confined to a cavity-receiver and laden with carbon particles that serve simultaneously as radiant absorbers and nucleation sites. A 5 kW reactor prototype (Fig. 3), tested at PSI's solar furnace and ETH's high-flux solar simulator with power flux intensities exceeding 3500 kW/m2, yielded 67% chemical conversion of CH4 to H2and C(gr) at 1600 K and 1 bar. Carbon formed was of nano-filamentary nature (Fig. 4). The proposed solar hybrid chemical process conserves natural gas, reduces CO2 emissions, and provides a transition path to solar hydrogen.

Project-related Publications