Thermochemical separation of O2 from gas mixtures
Funding source: external page EU-ERC, external page Swiss Federal Office of Energy
Partners: external page PSI
Postdoc: Dr. Ronald Michalsky
Background – This project deals with the thermochemical splitting of CO2 and H2O via metal oxide redox cycles, which has the potential of reaching high solar-to-fuel energy conversion efficiencies. In a first endothermic solar step, a metal oxide is thermally reduced at low partial pressure of oxygen (pO2) and high temperature using concentrated solar radiation as the energy source of process heat. In the second exothermic non-solar step, the reduced metal oxide is re-oxidized at a lower temperature with CO2 and H2O to produce syngas. A critical drawback of such a cycle is the inert gas consumption during the reduction step to lower the pO2 for shifting the thermodynamic equilibrium towards lower operating temperatures. This, in turn, requires separation of O2 from the offgas for recycling the inert carrier gas and closing the material cycle. Since solar thermochemical cycles inherently suffer from heat losses, it would be beneficial to utilize an oxygen separation technology driven by low-grade process heat, i.e., solar-thermal waste heat, without penalizing the solar-to-fuel energy conversion efficiency. Thermochemical O2 separation using perovskite redox materials enable low operating temperatures and the possibility to produce high-purity inert gas. The whole process is shown with Figure 1.
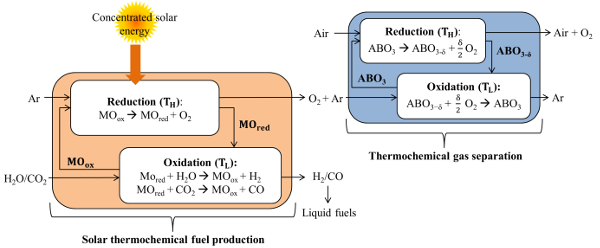
Scope – This research project proposes to utilize a dual approach to the rational design of perovskite redox materials by combining computational materials science and experimental characterization methods. This approach is aimed at investigating the structural properties of perovskites during redox reactions, while designing compositions that allow for low-temperature and high-efficiency thermochemical separation of O2 and thermochemical splitting of H2O/CO2.
Project-related Publications