SNF-Project Hydrogen Production by In-situ Formation and Hydrolysis of Zn Nanoparticles
Partner: Particle Technology Laboratory
Funding source: ETH-Zurich TH-Project
Background – The production of solar hydrogen via the Zn/ZnO water-splitting thermochemical cycle is considered, consisting of a 1st-step solar endothermic dissociation of ZnO and a 2nd-step non-solar exothermic hydrolysis of Zn :
1st step dissociation (solar): ZnO → Zn + 0.5 O2 (1)
2nd step hydrolysis (non-solar): Zn + H2O → ZnO + H2 (2)
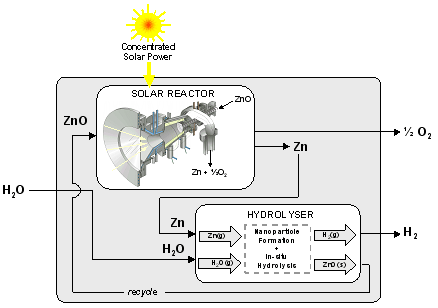
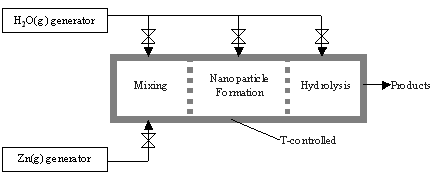
This project focuses on a novel combined process for the efficient execution of the 2nd step, Eq. (2), that encompasses the formation of Zn-nanoparticles followed by their in-situ hydrolysis for H2 generation. The advantages of using Zn-nanoparticles are 3-fold: 1) their inherent high specific surface area augments the reaction kinetics, heat transfer, and mass transfer; 2) their large surface to volume ratio favors complete or nearly complete oxidation; and 3) their entrainment in a gas flow allows for simple, continuous, and controllable feeding of reactants and removal of products.
Key objectives
- Thermodynamic and kinetic analysis of the combined nanoparticle formation + hydrolysis, for the purpose of determining the constrains for the design and efficient operation of the chemical reactor.
- Design and fabrication of a novel aerosol reactor featuring 3 temperature-controlled zones for the Zn-evaporation, steam-quenching, and Zn/H2O-reaction.
- Experimental demonstration of the combined process of Zn-nanoparticle formation and in-situ hydrolysis; optimization by parametric study.
- Theoretical modeling using coupled state-of-the-art fluid and particle dynamics accounting for nucleation, condensation, coagulation, surface reactions, and sintering; validation using experimental data.
Project-related Publications