CO2 Capture from Atmospheric Air using the CaCO3-CaO cycle
Motivation
- Air remediation: IPCC scenarios predict that CO2 capture from power-plant flue gases will not be enough to stabilize CO2 concentration in the atmosphere and additional CO2 capture from ambient air will become necessary in view of increasing emissions derived from transportation and other distributed sources.
- Emissions: Use of solar energy for process heat eliminates CO2 emissions produced during the energy-intensive capture process.
- Logistics: CO2 capture plant located next to the final storage site eliminates the need for CO2 transportation
Background – A novel solar thermochemical carbonation-calcination cycle for the capture of CO2 directly from air is depicted in Figure 1. The concentrated solar energy serves as the source of high-temperature process heat. The theoretical net energy requirements are estimated to be 2.5 MJ/mol CO2 captured.
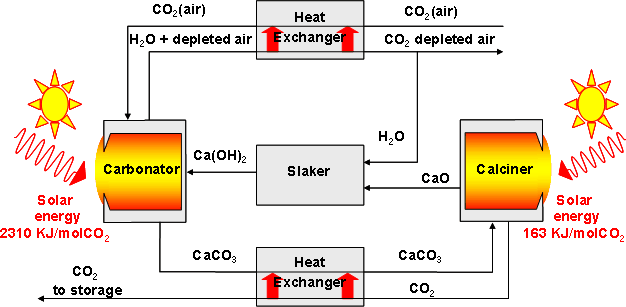
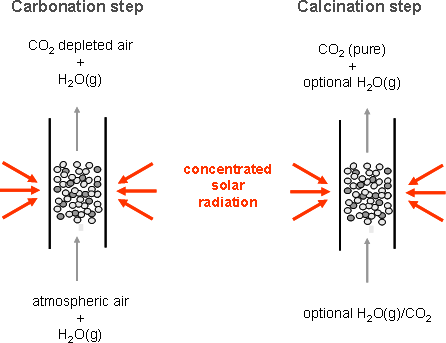
The solar reactor concept is illustrated in Fig. 2. It features a fluidized bed that serves the functions of both the carbonator and calciner, eliminating the need for transportation of solids.
- Carbonation step:
ambient air and steam is the fluidizing gas, CaO is transformed to CaCO3,and CO2-depleted air leaves the reaction site - Calcination step:
H2O or CO2 is the fluidizing gas, CaCO3 is transformed to CaO, and pure CO2 leaves the reaction site
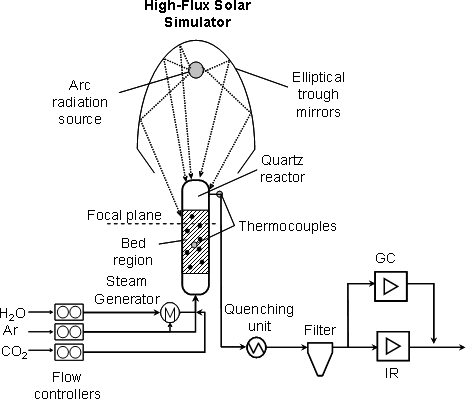
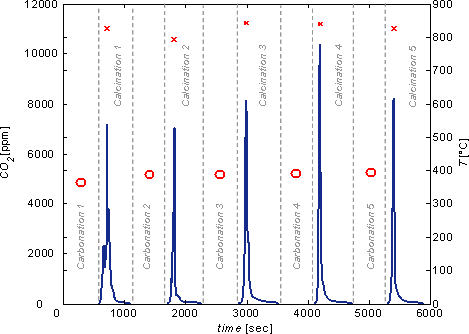
A laboratory-scale solar fluidized bed reactor system shown on Fig. 3 was tested for performing both steps of a carbonation-calcination thermochemical cycle for the removal of CO2 from ambient air using solar energy. Five consecutive cycles were experimentally demonstrated in a high-flux solar simulator. During all carbonation steps, CO2 was practically completely removed from air since the off-gas contained less than 1 ppm CO2 (Fig.4). During all calcination steps, CO2 was released until reaction reached completion after about 500 s. The fluidized-bed system proved to be a suitable reactor concept for effecting both steps of the proposed thermochemical cycle for capturing CO2 from ambient air.
Objectives – Investigate and experimentally demonstrate the thermochemical cycle for CO2 capture from air using concentrated solar power.
The research work includes:
- Thermodynamic analyses of the proposed solar thermochemical cycle.
- Exergy analysis for the open-material cycle that captures CO2 from air while co-producing H2.
- Screening and comparison of the different sorbents for CO2 capture from ambient air.
- Kinetic analysis of the carbonation reactions by thermogravimetry.
- Design, fabrication, and testing of a laboratory scale solar reactor system for performing carbonation-calcination thermochemical cycle.
Project-related Publications