Fuels from Sunlight and Air
Demonstration of the entire process chain to carbon-neutral fuels
- Link to Nature paper “Drop-in fuels from Sunlight and Air” (external page SharedIt link, external page DOI)
13-06-2019
For the first time, liquid hydrocarbon fuels are being thermochemically produced from concentrated sunlight and ambient air. Researchers of the Professorship of Renewable Energy Carriers at ETH Zurich developed the technologies and demonstrated the entire process chain under real field conditions in a solar mini-refinery system mounted on the roof of the ETH-Machine Laboratory. Using concentrated solar radiation, a high-temperature solar reactor splits CO2 and H2O extracted directly from air and produces syngas – a specific mixture of H2 and CO – which is finally processed into liquid hydrocarbons such as methanol or kerosene. The successful operation of the solar demonstration plant represents a crucial milestone towards the production of carbon-neutral synthetic fuels, which release only as much CO2 during combustion as was previously extracted from the air. These drop-in fuels are compatible with the worldwide existing infrastructures for fuel distribution, storage and utilization, and can particularly contribute to sustainable aviation and shipping. Two ETH spin-offs, external page Climeworks and external page Synhelion, are further developing these technologies for commercialization.
How does the solar mini-refinery function?
external page Here is an animation explaining the entire process chain (in Deutsch)
external page Here is an animation explaining the entire process chain (in English)
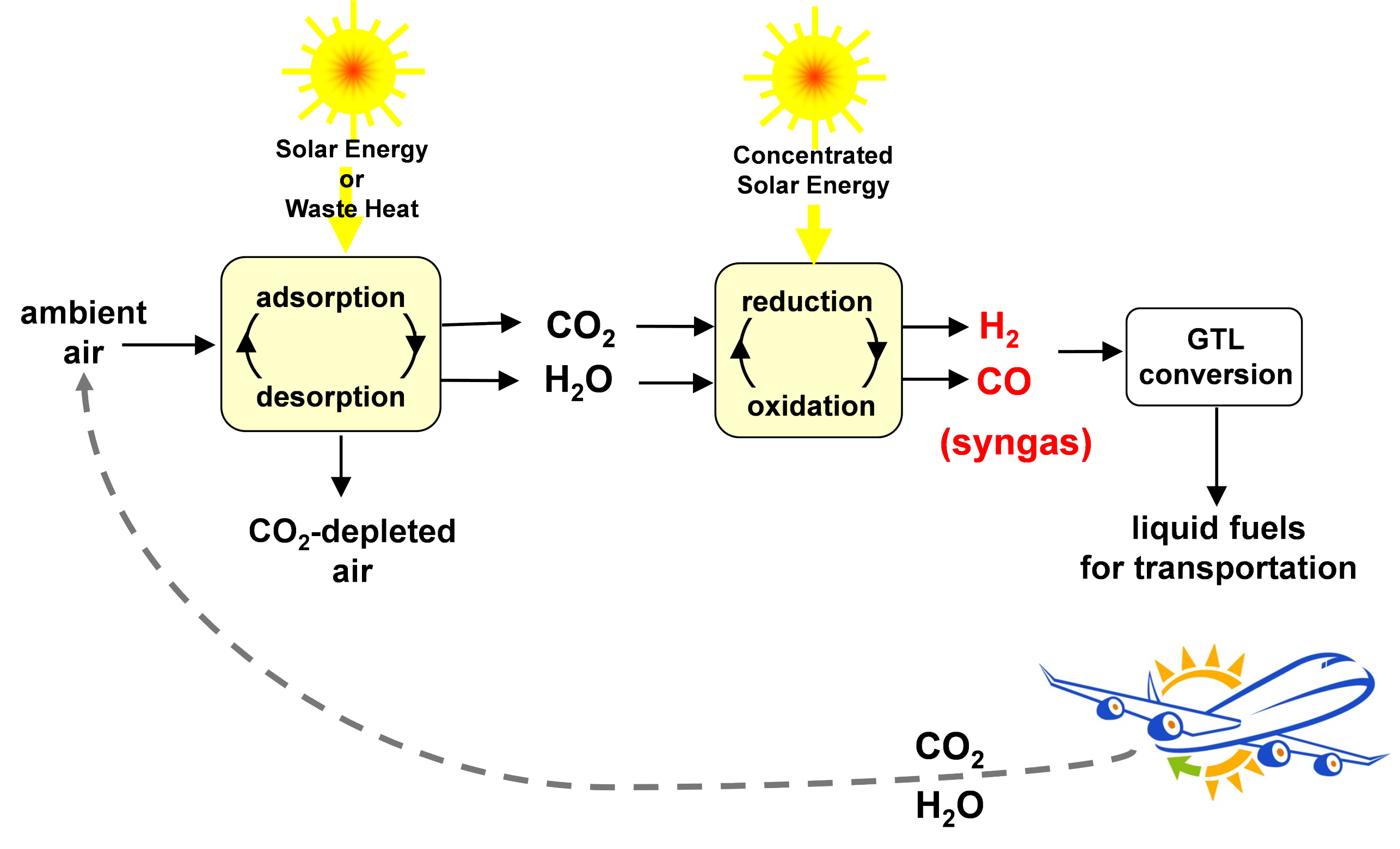
The solar-driven process chain utilizes the whole solar spectrum and inherently operates at high temperatures, and as such provides a favorable thermodynamic path to solar fuels production with high reaction rates and energy efficiencies [1]. The system design serially integrates three thermochemical conversion units (Figure 1):
- the Direct Air Capture unit which co-extracts CO2 and H2O from ambient air;
- the Solar Redox unit which converts CO2 and H2O to a desired mixture of CO and H2 (syngas);
- the Gas-to-Liquid unit which converts syngas to liquid hydrocarbons.
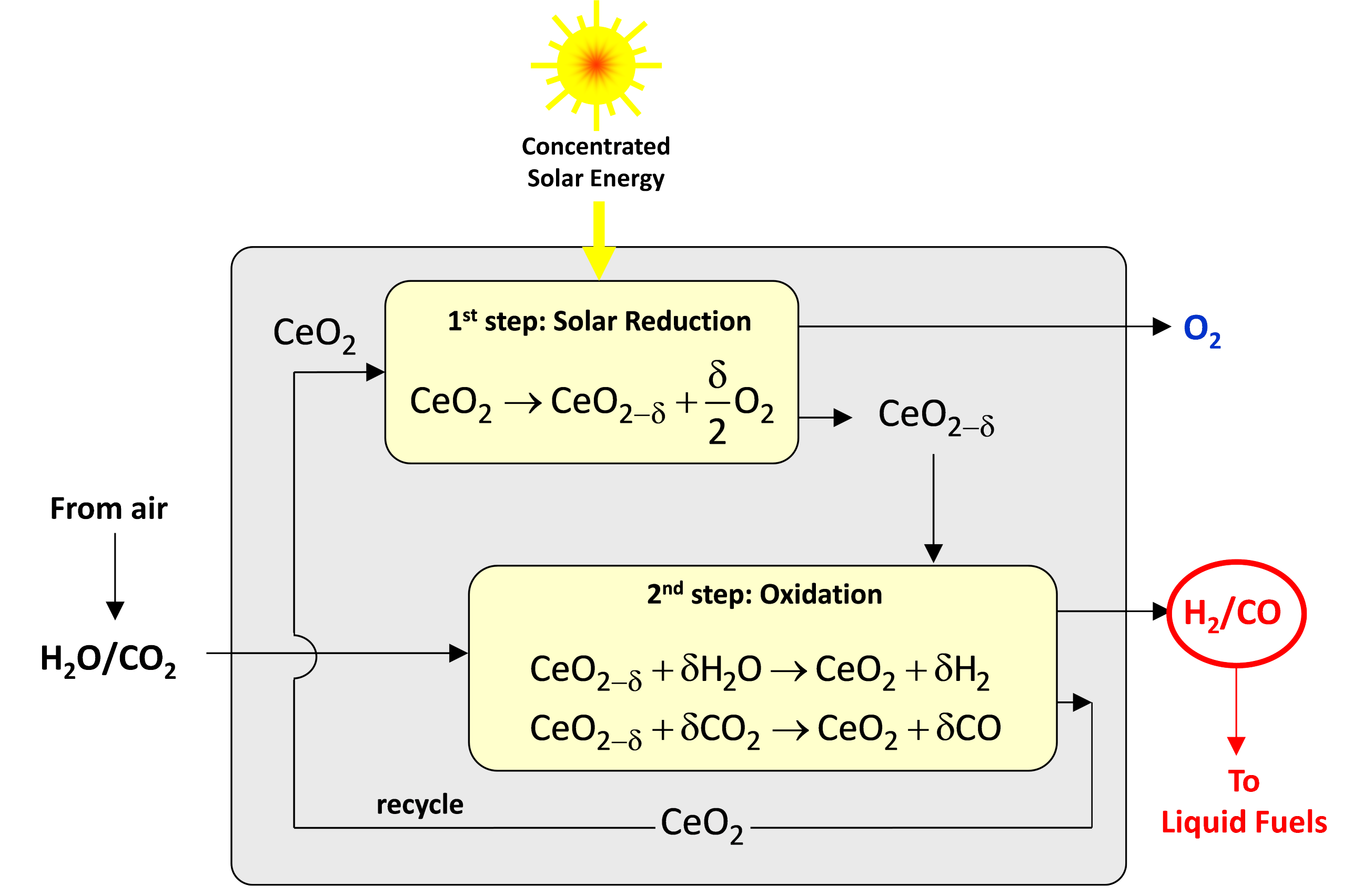
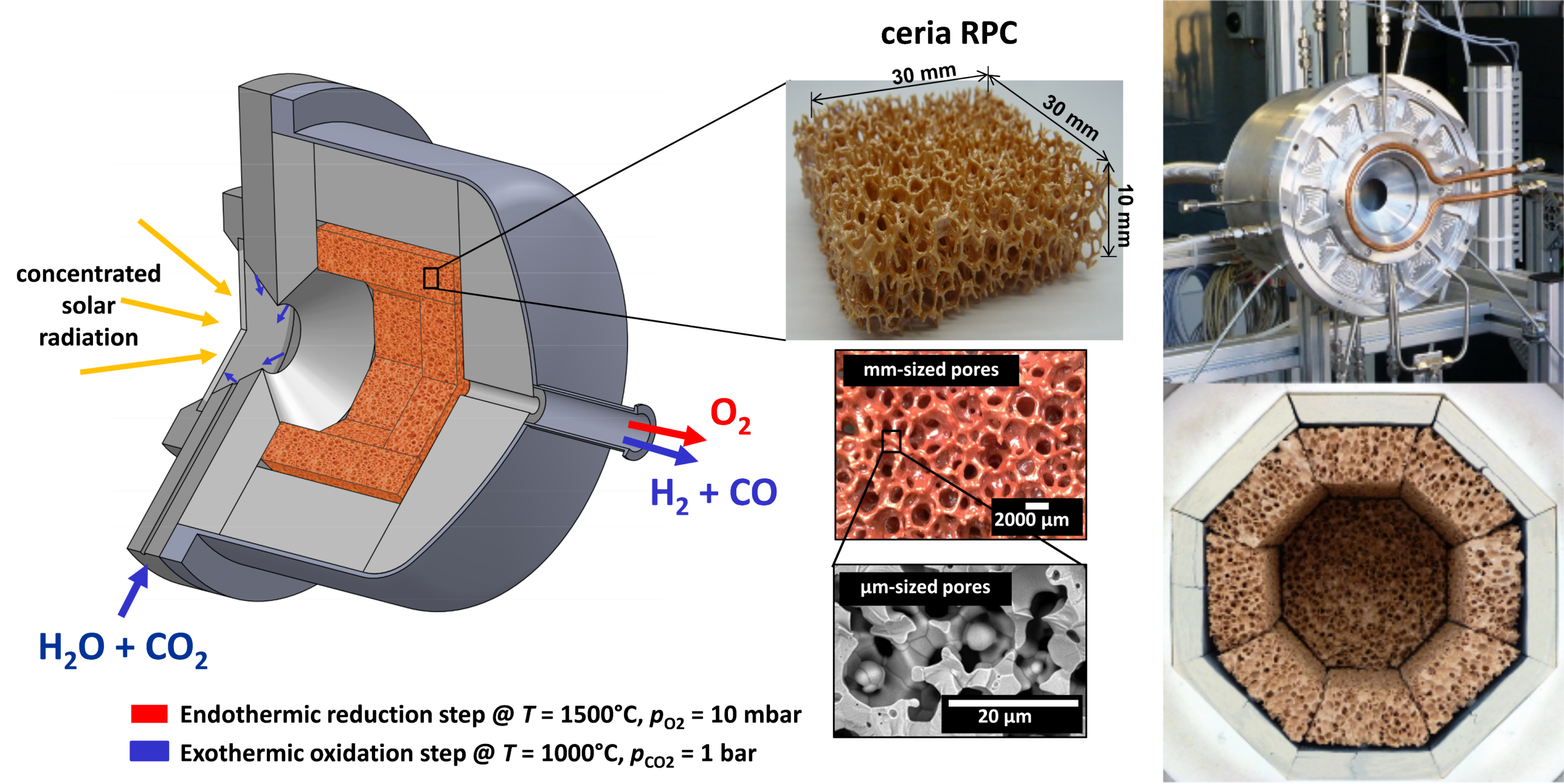
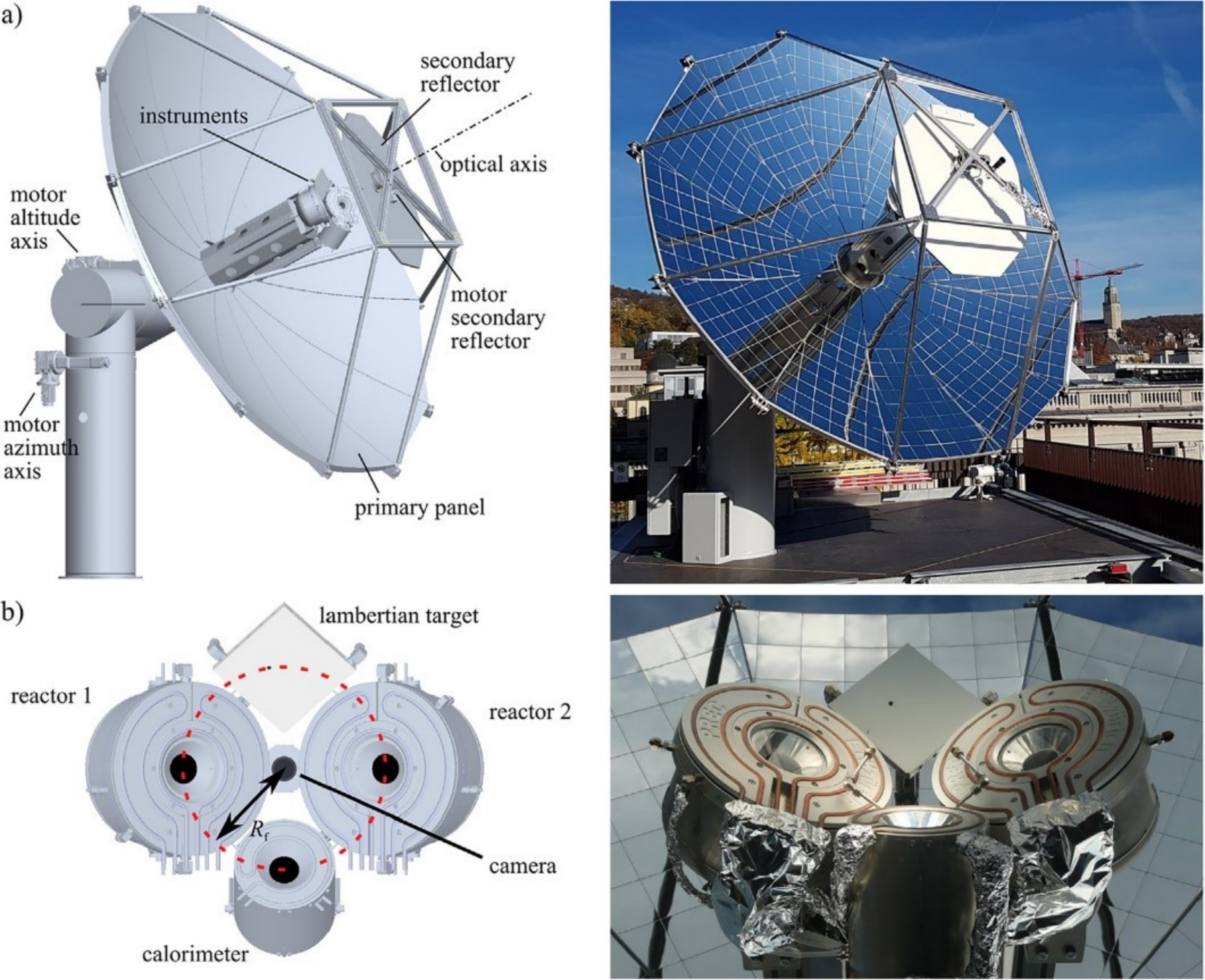
The Direct Air Capture unit is based on an adsorption-desorption cyclic process applied to an amine-functionalized sorbent to concurrently extract CO2 and H2O directly from ambient air [2]. Both CO2 and H2O are then fed to the Solar Redox unit. The Solar Redox unit is based on the thermochemical splitting of CO2 and H2O via a reduction-oxidation (redox) cyclic process using non-stoichiometric ceria (Figure 2). The redox cycle comprises 2 steps: In the first endothermic step, ceria is thermally reduced to generate O2 using concentrated solar energy as the source of high-temperature process heat. In the second exothermic step, the reduced ceria is re-oxidized with CO2 and H2O to generate a desired mixture of CO and H2 (syngas). Thus, ceria is not consumed and the net reactions are the thermolysis of CO2 and H2O: CO2=CO+½O2 and H2O=H2+½O2. The solar reactor [3] consists of a cylindrical cavity-receiver containing a reticulated porous ceramic (RPC) structure made of ceria that is directly exposed to the high-flux solar irradiation (Figure 3). Since only the endothermic reduction step requires a solar input, two identical solar reactors are employed for performing both redox reactions simultaneously by alternating the concentrated solar input between them (Figure 4). Finally, syngas is sent to the Gas-to-Liquid unit for processing into liquid hydrocarbons such as methanol or kerosene.
Towards industrial implementation
Two ETH spin-offs are further developing these technologies for industrial implementation: external page Climeworks commercializes the technology for direct air capture, while external page Synhelion commercializes the technology for solar fuel production. The technologies can be scaled up to produce carbon-neutral transportation fuels at the global scale without the need to replace the worldwide existing transportation infrastructures. The scale-up development is being pursued by the EU-project external page SUN-to-LIQUID. In the framework of this project, the ETH’s solar reactor is operated in a concentrating solar tower configuration (Figure 5) and delivers solar-made syngas to a Fischer-Tropsch unit. The solar-tower fuel plant now succeeded to demonstrate the first synthesis of solar kerosene from H2O and CO2 (Press release SUN-to-LIQUID).
Figure 1. Schematic the entire process chain to CO2-neutral hydrocarbon fuels using sunlight and air. The system design serially integrates three thermochemical conversion units: 1) the direct air capture unit which co-extracts CO2 and H2O from ambient air; 2) the solar redox unit which converts CO2 and H2O to a desired mixture of CO and H2 (syngas); 3) the gas-to-liquid (GTL) unit which converts syngas to liquid hydrocarbons. These are carbon-neutral synthetic fuels because they release only as much CO2 during their combustion as was previously extracted from the air for their production.
Figure 2. Schematic of the 2-step thermochemical cycle for splitting CO2/H2O into separate streams of CO/H2 and O2 via reduction-oxidation (redox) reactions using non-stoichiometric ceria (CeO2). In the first endothermic step, ceria is thermally reduced to generate O2 using concentrated solar process heat. In the second exothermic step, the reduced ceria is re-oxidized with CO2 and H2O to generate CO and H2, respectively. δ denotes the nonstoichiometry – the measure of the redox extent and thereby the fuel yield per cycle [1].
Figure 3. Schematic of the solar reactor configuration for splitting CO2 and H2O. It comprises a cavity-receiver containing a reticulated porous ceramic (RPC) structure made of ceria directly exposed to high-flux solar irradiation. Red arrow: endothermic reduction generating O2 is performed using concentrated solar energy. Blue arrow: exothermic oxidation with CO2 and H2O generating CO and H2 (syngas). Inset: infiltrated ceria RPC with dual-scale porosities in the mm and μm ranges for enhanced heat and mass transport. b) Photographs of the solar reactor, showing the front face of the solar reactor with the windowed aperture and its interior containing the RPC structure. Extracted from Ref [3].
Figure 4. Schematics and photographs of the concentrating solar system: a) 3D rendering of the primary two-axis sun-tracking parabolic concentrator coupled to a secondary rotating flat reflector. This optical configuration enables to alternate the focus between the two solar reactors while making uninterrupted use of the incoming sunlight. b) solar reactors, calorimeter, and Lambertian target for solar radiative flux measurements (3,000 suns concentration). The dashed circle indicates the focal point trajectory during rotation of the secondary reflector. Extracted from Ref. [4].
Figure 5. Photograph of the external page SUN-to-LIQUID solar fuel processing plant located at IMDEA-Energy, Madrid. A field of sun-tracking heliostats focusses the direct solar irradiation onto the solar reactor positioned at the top of the tower. The solar reactor co-splits CO2 and H2O via the ceria-based thermochemical redox cycle and produces a desired mixture of CO and H2 (syngas), which is finally processed via Fischer-Tropsch synthesis to kerosene. This solar fuel plant now succeeded to demonstrate the first synthesis of solar kerosene from H2O and CO2
Press release SUN-to-LIQUID
Acknowledgements – The research on solar fuels is being funded by the Swiss Federal Office of Energy (Grant No. SI/501213-01), the Swiss National Science Foundation (Grants No. 200021-162435 and No. 206021_170735), the Swiss State Secretariat for Education, Research and Innovation (Grant No. 15.0330), the EU’s Horizon 2020 Research and Innovation Program (Project SUN-to-LIQUID – Grant No. 654408), and the European Research Council under the European Union’s ERC Advanced Grant (SUNFUELS – Grant No. 320541).
Selected References
(List of project-related publications)
- Romero M., Steinfeld A., “Concentrating Solar Thermal Power and Thermochemical Fuels”, Energy & Environmental Science, Vol. 5, pp. 9234–9245, 2012. external page DOI: 10.1039/c2ee21275g
- Wurzbacher J., Gebald C., Piatkowski N., Steinfeld A., “Concurrent Separation of CO2 and H2O from Air by a Temperature-Vacuum Swing Adsorption/Desorption Cycle”, Environmental Science & Technology, Vol. 46, pp. 9191-9198, 2012. external page DOI: 10.1021/es301953k
- Marxer D., Furler P., Takacs M., Steinfeld A., “Solar thermochemical splitting of CO2 into separate streams of CO and O2 with high selectivity, stability, conversion, and efficiency”, Energy & Environmental Science, Vol. 10, pp. 1142-1149, 2017. external page DOI: 10.1039/c6ee03776c
- Dähler F., Wild M., Schäppi R., Haueter P., Cooper T., Good P., Larrea C., Schmitz M., Furler P., Steinfeld A., “Optical design and experimental characterization of a solar concentrating dish system for fuel production via thermochemical redox cycles”, Solar Energy, Vol. 170, pp. 568-575, 2018. external page DOI: 10.1016/j.solener.2018.05.085
- Schäppi R., Rutz D., Dähler F., Muroyama A., Haueter P., Lilliestam J., Patt A., Furler P., Steinfeld A., “Drop-in fuels from sunlight and air”, Nature, in press 2021. external page DOI: 10.1038/s41586-021-04174-y
- Zoller S., Koepf E., Nizamian D., Stephan M., Patané A., Haueter P., Romero M., Gonzalez-Aguilar J., Lieftink D., de Wit E., Brendelberger S., Sizmann A., Steinfeld A., “A solar tower fuel plant for the thermochemical production of kerosene from H2O and CO2”, Joule, Vol. 6, pp. 1606-1616, 2022. external page https://doi.org/10.1016/j.joule.2022.06.012
Links
Press release ETH (13-6-2019) in English
Press release ETH (13-6-2019) in Deutsch
Fuels from Sunlight and Air - Press Coverage 2019
Nature Paper - Press Coverage 2021
Joule Paper - Press Coverage 2022
ETH-News interview (English/Deutsch) 2021
Download Video Clip - Fuels from sunlight and air (MP4, 31.1 MB)
Download Animation - Fuels from sunlight and air (MP4, 271.4 MB) in Deutsch
Download Animation - Fuels from sunlight and air (MP4, 421.1 MB) in English
Images and Videos - Fuels from sunlight and air
Press release SUN-to-LIQUID (13-6-2019)